Search
Physiology of Birth Asphyxia
Statement

This statement is not a peer reviewed guideline.
This statement has been developed to provide additional information regarding the physiology of birth asphyxia. It should only be used as a guide to these principles . The information is supplementary and has not undergone the same peer review process required of ANZCOR guideline development.
Introduction

The newborn is not an adult, nor a child.
In people of all ages, death can occur from a failure of breathing and / or circulation. The interventions required to aid recovery from these situations are termed resuscitation.
In the absence of circulation or breathing, the interventions required for resuscitation may include the maintenance of respiration using artificial ventilation and the maintenance of an artificial circulation using chest compressions. The combination of chest compressions with artificial ventilation is called cardiopulmonary resuscitation or CPR. Occasionally, when the situation warrants it, additional measures may be required, including the use of artificial airways, defibrillation (almost unheard of in neonates), and the administration of fluid or drugs.
Babies do not suffer from the same diseases and problems that cause cardio-respiratory collapse in adults or children. Newly born babies are not small adults, nor just small children. Their physiology and anatomy are different and so are the reasons why resuscitation may be required.
The resuscitator must approach a newborn differently from a collapsed adult or child.
Difference between Adult and Newborn Resuscitation

In adults, cardio-respiratory collapse is usually a cardiac event and caused by a sudden failure of circulation. The most common cause of cardiac arrest in adults is the potentially lethal arrhythmia ventricular fibrillation, as a result of myocardial infarction or myocardial ischaemia. Following primary cardiac arrest breathing stops because oxygen is no longer being delivered to the respiratory centre in the brain stem. To manage adult cardiac arrest, the rescuer will attempt to provide a temporary artificial circulation of oxygenated blood to the heart and brain using chest compressions combined with lung ventilation (cardiopulmonary resuscitation or CPR). The emphasis in adult CPR is on chest compressions over ventilations. With adult cardiac arrest the circulation immediately ceases but the oxygen level in the left ventricular blood does not fall, because blood is no longer moving to areas of the body where oxygen levels are low. The aim of adult CPR is to establish an adequate coronary perfusion pressure (the difference between aortic diastolic pressure and right atrial pressure; animal studies suggest it needs to be at least 15mmHg) and push LV blood into the ascending aorta. Whenever chest compressions are interrupted the coronary perfusion pressure plummets and takes about 6 chest compressions to return to the pre-interruption level. Having established a temporary circulation of oxygenated blood using CPR, the resuscitator attempts to identify the problem and provide appropriate treatment. This often requires a defibrillator, and possibly drugs.
In contrast to adults, the need for resuscitation in newly born infants is almost always secondary to hypoxia and a failure of breathing (apnoea), a respiratory arrest. Primary cardiovascular collapse is extraordinarily rare. Newborn resuscitation is always focussed on the initiation and maintenance of ventilation. The neonatal myocardium has a great resilience to ischemia and can keep pumping using (less efficient) anaerobic mechanisms for a number of minutes although at a lower than normal heart rate. Unlike adults, because left ventricular blood in the neonate is still being pumped forward by the beating heart, the oxygen level in that blood rapidly falls as it is not being replenished by blood exposed to effective ventilation or a functioning placenta. If ventilation is restored or established the first sign will be a rise in heart rate. Despite the resilience of the neonatal myocardium, any reserve will eventually be exhausted. If the heart rate drops below 60 chest compressions should be added to the ventilations. The neonatal algorithm of 3 compressions: 1 ventilation emphasises ventilation over compressions, as in most cases the heart rate will rise rapidly once adequate ventilation is restored. The exact age, postnatally, to change from newborn ventilation compression ratios to a paediatric one (15:2) is unknown. Unless the arrest is thought to be of a purely cardiac aetiology, the newborn ratio should be used until the newborn is outside the birthing environment. The compression ventilation ration for cardiac cases or older children is 15:2.
Hypoxia

The most important changes at birth involve the discontinuation of feto-placental circulation and the initiation of pulmonary ventilation. The principal danger for the fetus during this transition is the risk of hypoxia. Feto-placental oxygenation may be impaired in utero prior to the disruption that will occur at birth itself. Some infants may become profoundly hypoxic in utero before pulmonary oxygenation can be established. A common response to this stress, in term babies, is the passage of meconium into the amniotic fluid prior to delivery.
The response to hypoxia
The figure below shows the response of fetal mammals to acute hypoxia (cord clamping in the absence of pulmonary ventilation) in utero. Similar responses to acute hypoxia are apparent in the human fetus, and may occur in utero or after birth. Clinical examples of this scenario would be perinatal obstetric conditions such as uterine rupture, placental abruption, cord avulsion or cord compression during shoulder dystocia.
Figure: Induced hypoxia in newborn mammals. Note that birth may occur at any point such as 1, 2 or 3. The initial status of a baby may not indicate where in this process the physiology is progressing. Only the response to resuscitation will retrospectively indicate the initial status of the baby (primary apnoea, gasping respiration, terminal apnoea).
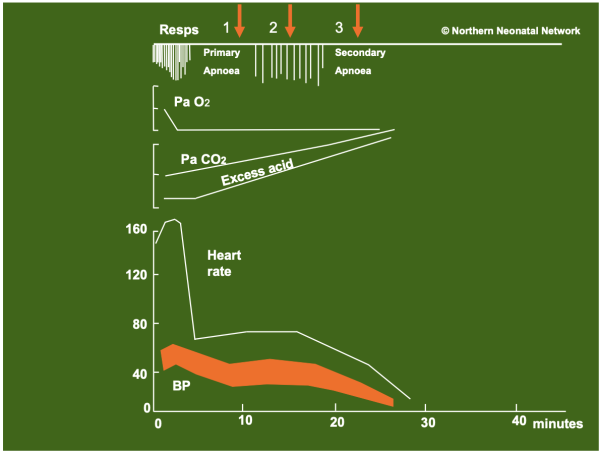
Primary apnoea

At the onset of hypoxia fetal breathing movements become deeper and more rapid. As arterial oxygen level falls the brain becomes hypoxic. Muscle tone diminishes and, within a few minutes, regular breathing movements cease as the brainstem centre responsible for controlling respiration is unable to function due to lack of oxygen. With termination of breathing the fetus enters a period known as primary apnoea. Although initially heart rate remains reasonably constant, or briefly rises, it soon falls to about half its normal rate. This bradycardia is due to an initial increase in vagal tone but persists because, due to lack of oxygen, heart muscle is increasingly required to contract using anaerobic metabolism.
Despite bradycardia, blood pressure is maintained, initially by two mechanisms: vasoconstriction shuts down the circulation to all but the most vital areas of the body (heart and brain) and blood supply to the gut, kidneys, muscles and skin diminishes. The slow heart rate allows greater time for the ventricles to refill in diastole and stroke volume (volume pumped from the heart with each contraction) increases.
Overall, cardiac output (the product of heart rate and stroke volume) does decrease but the fall is not as great as would be expected from the decrease in heart rate. Although circulation is maintained to the organs most important for immediate survival, release of lactic acid as a by-product of anaerobic metabolism in poorly perfused tissues causes metabolic acidosis and ongoing biochemical deterioration. The majority of term infants born during primary apnoea respond well to tactile stimulation and having ventilation assisted by the provision of a patent airway. Brain oxygenation will be rapidly restored and therefore induce normal breathing.
Gasping

If the hypoxic insult continues, after a variable period of time, primitive spinal centres, released from the inhibition of higher breathing centres, produce shuddering, exaggerated, irregular gasps at a rate of about 12 per minute. The heart rate continues to decrease.
A variable time may elapse before this unconscious gasping activity begins. Anaesthetics and drugs such as maternal opiates can delay the onset of gasping (i.e. increase the duration of primary apnoea) as well as reduce the duration of the subsequent gasping period.
If these gasps successfully aerate the lungs then, because the circulation is still functioning, oxygenated blood will be transported to the coronary arteries and the heart rate will rapidly increase. This will improve the circulation and will facilitate the transport of oxygenated blood to the brain and respiratory centre. Once the respiratory centre is re-oxygenated it will begin to function again, regular breathing will gradually start and gasping will gradually cease.
Terminal apnoea

If the period of gasping occurs in utero because of intrauterine hypoxaemia or, after birth, fails to aerate the lung and provide oxygenation, hypoxic damage to the brain and heart will continue. Gasping will become weaker and eventually cease. The baby will progress into terminal apnoea. Although the newborn heart is resilient to hypoxic stress (a consequence of its large glycogen stores) this resilience is finite. The heart rate and blood pressure will start to fall and eventually become undetectable.
Apnoea at birth

A baby who is not breathing within a minute or two of birth may be either in a) primary apnoea or b) terminal apnoea having gone through a period of gasping in utero. Clinically, these phases are indistinguishable, and one should assume the baby is in terminal apnoea. Resuscitation with ventilation should commence without delay.
In most babies born during primary apnoea, tactile stimulation and enabling ventilation by providing a patent airway will rapidly restore normal breathing. Many of these babies will be able to ‘resuscitate’ themselves provided the airway is open. After a pause, the baby will take the first of a series of gasps that will aerate the lung, provide oxygenation to the heart and brain, and initiate the return of normal breathing.
A baby born in terminal apnoea will show no further respiratory effort and will die without intervention and may die despite it. It is possible that the application of effective artificial lung inflation may be enough to produce a rapid recovery, provided there is still a circulation functioning sufficiently to bring oxygenated blood back to the heart.
In a few babies the situation may have progressed to a stage where the circulation is no longer functional and is unable to deliver oxygenated blood from the lungs to the heart despite adequate lung inflation. In this situation recovery can still occur, in some cases, if lung ventilation is combined with a period of chest compressions. These compressions can successfully deliver a small quantity of oxygenated blood to the heart, provided the heart is still able to respond.
The behaviour exhibited as the baby improves is an indication as to how far the baby has progressed into terminal apnoea. Babies who have been in terminal apnoea do not cough or gasp until the circulation is restored and exhibit a period of gasping before normal breathing movements make their appearance. A period of intermittent positive pressure ventilation (IPPV) may be required until normal breathing is established.
References

- Cross KW. Resuscitation of the asphyxiated infant. British Med Bull 1966; 22: 73-8
- Dawes G. Fetal and neonatal physiology: A comparative study of the changes at birth. Year Book Medical Publishers, Chicago 1968; Chapter 12: 141-59
- Godfrey S. Blood gases during asphyxia and resuscitation of fetal and newborn rabbits. Resp Physiol 1968; 4; 309-321
- Godfrey S. Respiratory and cardiovascular changes during asphyxia and resuscitation of foetal newborn rabbits. Quart J Exper Physiol 1968; 53: 97-118
- Hey E, Kelly J. Gaseous exchange during endotracheal ventilation for asphyxia at birth. J Obstet Gynaecol Br Commonw 1968; 75: 414-423
- Moore WMO, Davies JA. Response of the newborn rabbit to acute anoxia and variations due to narcotic agents. Br J Anaesth 1966; 38: 787-92
Referencing this guideline

When citing the ANZCOR Guidelines we recommend:
ANZCOR, 2025, Physiology of Birth Asphyxia , accessed 8 July 2025, https://www.anzcor.org/home/neonatal-resuscitation/physiology-of-birth-asphyxia/



